The Exponent Difference
Everything You Need, in One Place
Go Bigger, Faster
Novel Devices Require Novel Tests
Unique Multidisciplinary Expertise
Robust Failure Analysis Insights
Credentialed Talent at the Forefront of Innovation
Drive Safety & Compliance
With every advancement in medical device materials and technologies, understanding how they will perform, wear down, and interact with the human body becomes increasingly complex. Engaging our experts early in your product development can help you get ahead of risks, while positioning your devices to achieve FDA and EU compliance.
Biocompatibility
Address regulatory requirements while driving the safety of a new generation of medical and implantable devices.
Medical Device Corrosion
Understand how your product can corrode in the short and long term to improve performance and address vulnerabilities.
Biomechanical Support
Assess how your medical devices impact the people who use them to evaluate the potential for injuries, and create preventive solutions.
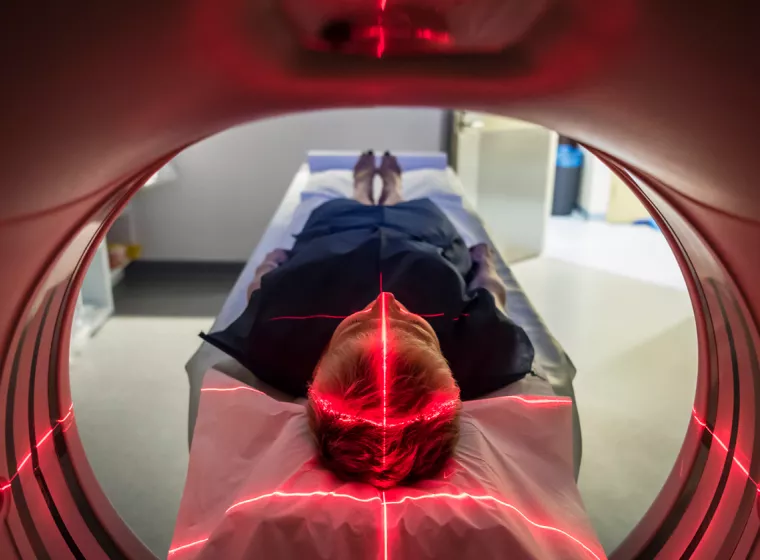
MRI Compatibility
Identify compatibility hazards, optimize your device designs for MRI environments, and meet regulatory labeling requirements.
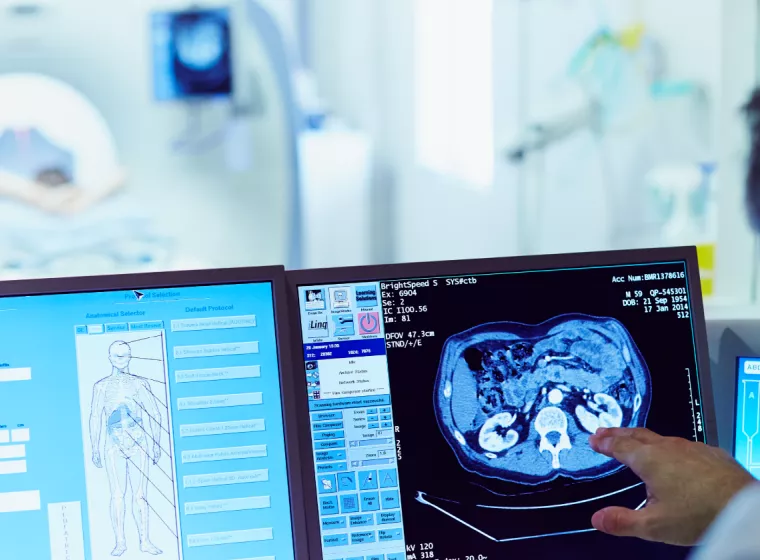
Retrieved Device & Tissue Analysis
Support pushing forward with your medical device development, clinical studies, and post-market surveillance through the insights gained from retrieved devices.
![Medical Devices, Implants & Surgical Tools [MCE]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-1013707860.jpg.webp?itok=PvunbsCo)
Biofluid Dynamics
Gain competitive edge by understanding the interactions that occur between biofluids and medical devices.
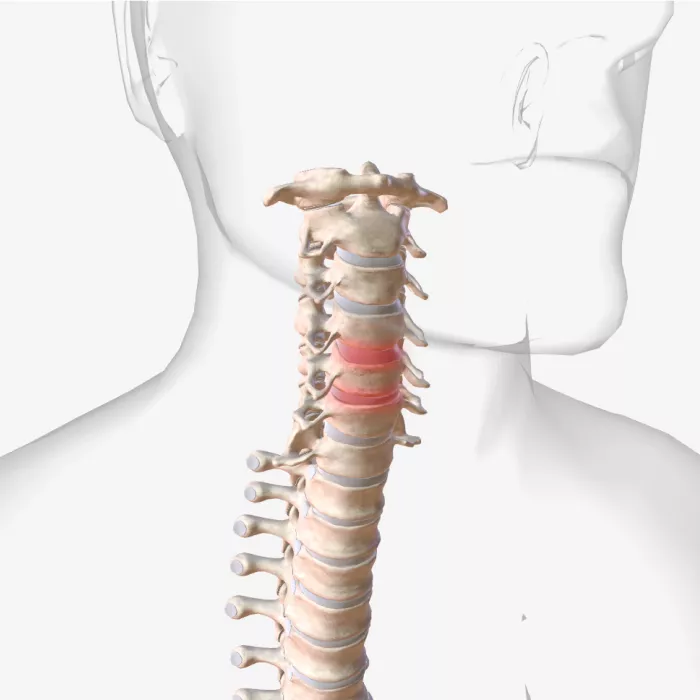
Anticipating Medical Device Lifespan & Impact
Get a clear understanding of how your medical devices may wear over time and the related impacts on the people who use them. Exponent offers extensive experience conducting medical device wear testing, establishing custom test programs, and providing data analysis insights to prepare your medical devices for the regulatory process. Our expertise includes conducting wear and durability testing of orthopedic, spine, and cardiovascular devices.

Expertise in Using Polymers in Medical Implants
Exponent is helping guide the use of polymer biomaterials in next-generation medical implants. Our experts have contributed to many publications, as well as "the book" on polyetheretherketone, known as PEEK (ASTM F2026). We also offer extensive experience on the analyses required for evaluating polyethylene (ASTM F648 and ASTM F2759, and the UHMWPE Biomaterials Handbook).

Innovation That Improves Outcomes
Medical devices are expected to perform under a range of conditions in and around the human body. From biocompatibility and corrosion assessments to finite element analysis and MRI compatibility, Exponent delivers essential insights to help understand device performance.

Bespoke Testing, Analysis, and Investigations
Exponent's unmatched team of scientists and engineers help clients across industries address complex molecular biology challenges with custom testing, evaluation, and risk mitigation.
Collaboration That Helps You See the Full Picture
Exponent is uniquely positioned to assemble a team tailored to your specific challenges. We bring together multidisciplinary experts to provide a broad perspective to help ensure relevant questions are addressed.
Hold Your Products to a Higher Standard
Exponent brings an unparalleled level of integrated expertise to every test and evaluation. Our commitment to scientific excellence is your competitive advantage.
Get in touch to discuss your unique needs.
![Battery Supply Chain Services [EECS]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-1411358298.jpg.webp?itok=Yd7-744o)
Battery Evaluations
Gain confidence with our cutting-edge evaluations and consulting.
![Vehicle Electronics & Electrical Systems [EECS]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-184621170.jpg.webp?itok=lE-1QQ8k)
Electric Vehicle Evaluations
Optimize the safety, reliability, and commercial viability of your electric vehicles.
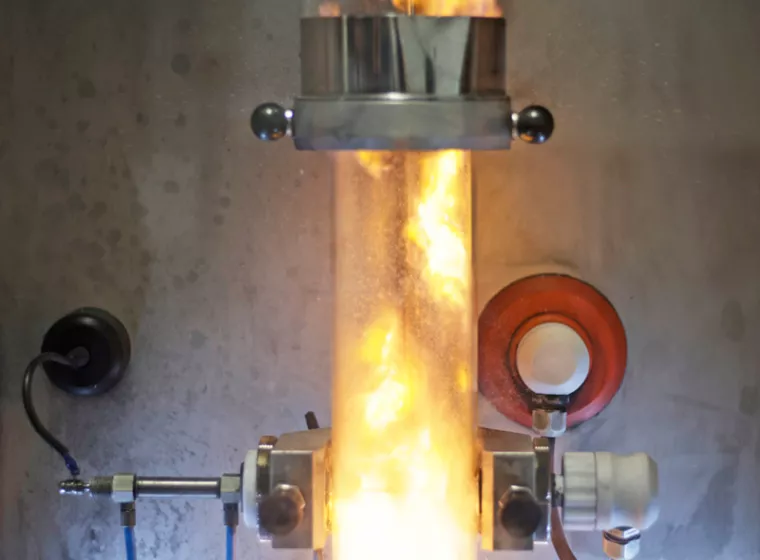
Fire & Flammability Testing
Assess fire and flammability risks with custom testing that goes above and beyond the standards.

Intentional Data Acquisition
Collect hard-to-acquire real-world data for products that go on or in human bodies.
Materials Evaluations
Build a strong foundation for your products with specialized materials evaluations.
![Mechanical Lab [ME]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-1340198475.jpg.webp?itok=u4zRNN5-)
Mechanical Evaluations
Understand your product's strengths and limitations with customized mechanical testing.

Transportation Evaluations
Quantify product performance, analyze system & component failures, and address claims.