August 25, 2022
RAS hardware, software & user synergy increases the value proposition early in the design process
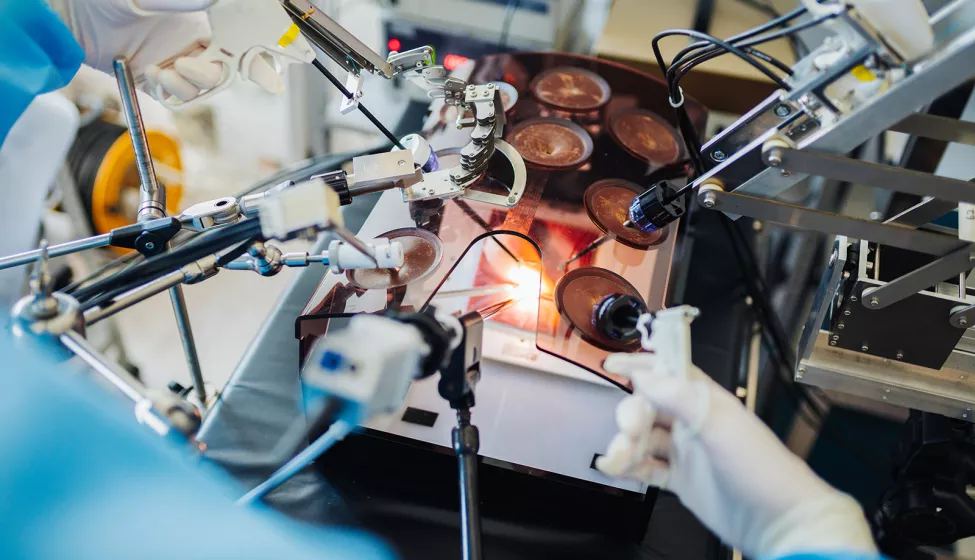
Robotic-assisted surgery (RAS) enables procedures akin to science fiction — like appendectomies performed through only a small incision hidden in the belly button.
To be successful, however, RAS requires sophisticated coordination between hardware, software, and user elements. A RAS system not only needs to help surgeons plan for and visualize procedures better than conventional surgical methods but also provide real-time feedback so that critical functions such as incision depth are gauged accurately.
Given benefits such as reduced risk of infection, speedier recovery times, and better patient outcomes, the demand for RAS is growing — but widespread adoption could be further accelerated. Some obstacles include the significant capital expenditure RAS requires and longer learning curves for surgeons and their teams. Additionally, complications related to hardware malfunctions, cybersecurity breaches, unreliable networks, or user feedback that misguides surgeons can drag down the overall value proposition of RAS.
To help mitigate these hurdles and reap the full potential of RAS, stakeholders ranging from reliability engineers to manufacturers to software companies have a key opportunity: to carefully account for hardware, software, and user components as early as possible in the design phase.
By using failure mode and effects analysis (FMEA) to identify, prioritize, and mitigate failure modes in the design process, stakeholders can improve RAS performance.
Hardware components
To increase the speed and precision of a surgical procedure, robotic hardware components need to be designed with flexibility in mind. This ensures they are adaptable to a range of procedures, environments, and patient anatomies. Miniaturizing hardware components to fit a variety of smaller ports is one way adaptability can be enhanced.
However, this introduces new challenges; for example, disinfection may be more difficult due to hard-to-clean nooks and crannies. Design engineers will want to consider shape/geometry and component modularity, as well as material selection. Optimizing these elements results in tools that can withstand the forces applied during surgery while maintaining their functionality and still serving many indications.
Other hardware challenges involve controller components and visualization tools, including cameras or imaging devices. The ability to incorporate pre-surgery images through augmented reality, for instance, is one way that RAS systems can benefit patients and surgeons. But how can designers ensure that cameras are precise, recording in real-time, and that augmented reality visualizations accurately reflect the body? And how can controller systems be designed to better notify, constrain, or guide users through system feedback if something is wrong with the force, depth, or location of an incision?
By using failure mode and effects analysis (FMEA) to identify, prioritize, and mitigate failure modes in the design process, stakeholders can improve RAS performance. Recreating surgical complications from intra-operative information the robot may store in real-time can further help stakeholders determine root causes of complications or optimal surgical approaches for specific patient subpopulations. The stored data may also be paired with clinical follow-up data to build evidence for the clinical and cost effectiveness of the RAS system.
Software security challenges
Connecting any system to a network involves risk, but RAS systems bring additional cybersecurity concerns. Unauthorized users might try to take control of the robot or stage a denial-of-service attack that slows or shuts down connectivity. This can disrupt real-time feedback, cause camera delays, and potentially result in patient harm. Confidential patient information may also be vulnerable, as well as trade secrets or other protected information about the device itself.
Intervening early in the design process can speed time to market and help ward off potential post-market threats. One way to build security into the design is to develop a threat model and perform a cybersecurity risk assessment. The STRIDE model is a useful tool for building a threat-risk model and classifying threats by category:
Spoofing: Using a false identity to gain unauthorized access to a system
Tampering: Loss of data integrity, for example, by unauthorized modification of data
Repudiation: Denial by users (legitimate or otherwise) that they performed specific actions or transactions
Information Disclosure: The unwanted exposure of confidential or private data
Denial of Service: Making a system or application unavailable for its intended use
Elevation of Privilege: When a user or other principal gains unauthorized access to an asset or function
To support building cybersecurity into medical device design, the Food and Drug Administration issued new draft guidance, "Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions." This draft document emphasizes the importance of risk assessment and threat modeling. Once finalized it may require manufacturers to provide a software bill of materials so companies can track different versions of operating systems, libraries, etc. to uncover cybersecurity vulnerabilities early in the design process.
User elements
RAS systems also create new device/system user challenges. For example, because the surgeon may only see what is visible through the camera — without additional imaging systems — and be seated away from the patient and surgical team, verbal and nonverbal communication (e.g., gestures, facial expressions) could be impeded. Coordination problems can also disrupt the RAS workflow, like the time-intensive set up of RAS equipment in the operating room.
How can communication channels and other user interface problems such as new postural stressors associated with visualization and control interfaces be identified and mitigated? While these issues add a layer of complexity to successful adoption, use-related risk analysis early in the product development process helps promote user-centered design. Methods include identifying the use-related characteristics of the intended users and use environments, outlining the tasks involved in relevant use scenarios, examining available sources for known use problems, and conducting usability research with representative users, such as simulated-use testing, to uncover additional use problems. Potential use problems can also be uncovered through the perception, cognition, and action (PCA) model:
Perception: What might happen if a user misperceives or does not perceive a signal from the user interface?
Cognition: What might happen if a user misinterprets, miscomputes, misremembers, etc. the output?
Action: What might happen if a user makes an unintended action, or does not make an intended action?
This model allows consideration of the clinical consequences of use problems and corresponding identification of user interface requirements. For example, is the controller alarm audible and distinct from other meaningful sounds? Is the controller alarm interpretable as "cutting too deeply"? Is the action that stops cutting available and achievable when needed?
RAS could soon be a vital part of the patient continuum. Increasing the value proposition of RAS early in the product development process can not only enhance precision and safety but also help expand access to minimally invasive procedures with improved patient outcomes.
How Exponent Can Help
With technical expertise spanning electrical and biomedical engineering, computer science, and human factors, we can help optimize RAS systems throughout the product life cycle via product design evaluation, material selection, and failure analysis, with a focus on how patient, surgical/clinical, and device/design factors influence clinical performance, including device-tissue interactions and user experience. We can also help improve the delivery, reliability, and security of RAS by considering, ranking, and mitigating or eliminating cybersecurity risks.


![User Research & Testing [HF]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-1338374024.jpg.webp?itok=bBXBoCIg)
![Medical Devices, Implants & Surgical Tools [MCE]](/sites/default/files/styles/cards_home_card/public/media/images/GettyImages-1182458826.jpg.webp?itok=z7lVBn-v)

