How can you accelerate the path to market for innovative new medical devices?
Clear design and development plans are critical for efficient medical device design, but many equipment manufacturers experience major schedule, resource, and expense challenges and fail to make it through validation. Exponent's electrical and biomedical engineers provide engineering expertise early in the medical device product development process and routinely provide the following support:
- Medical device testing, including accessory components and power management
- IEC60601 standards implementation and certification process preparedness
- Medical equipment and system lifecycle management support (including early design)
- Risk management requirements, such as hazard identification, risk acceptability criteria, and pre- and post-production risk analysis
- Failure root cause analysis for medical device manufacturers
- Remediation support with electromagnetic compliance re-qualification
Our Capabilities Are Unparalleled
With expertise in over 90 disciplines and hundreds of capabilities, tools, and methodologies — we get to the root of even the most complex challenges and give you the objective answers you need.
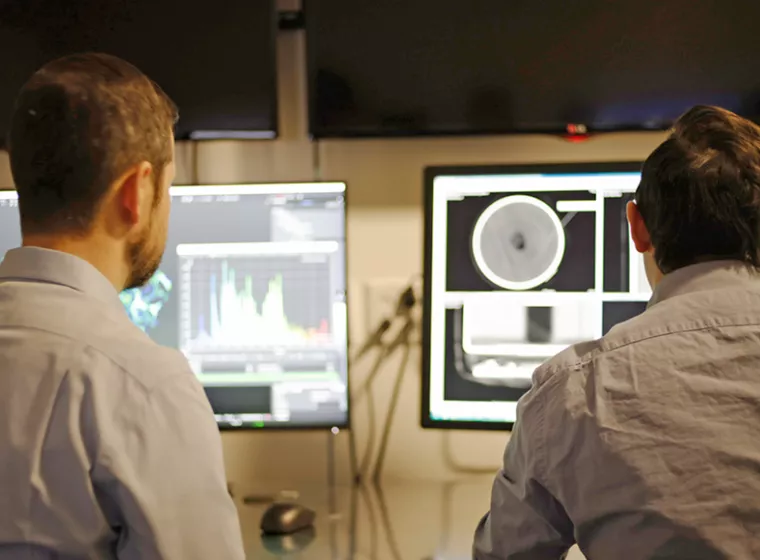
Software & Computer Systems Support
Insights and solutions for the design, development, and analysis of software prototypes, products, and platforms.

Systems & Controls
Critical systems and controls support, from missile guidance and ADAS technology to utility power generation and consumer electronics.

Electrical Devices & Consumer Products
Product design validation, risk assessment, product launch support, failure analysis, product recalls, and more.

Power & Energy
Multidisciplinary support for high-power energy systems of all sizes in a variety of applications.

Batteries & Energy Storage
Supercharge performance, reliability, and safety across all stages of the battery and energy storage product lifecycle.
Electrical Engineering & Computer Science Expertise for Intellectual Property
Leverage best-in-class electrical engineering and computer science consulting for IP challenges
Product Development
Expert electrical engineering and computer science consulting services for every stage of your product journey.
Technical Forensics
Failure analyses, predictive modeling, and accident reconstruction for valuable insights that help you prevent accidents and failures.
Experts
Our global and comprehensive expertise across industries gives us a deep understanding of current challenges, best industry practices, and the implications of emerging technologies.

Corporate Vice President, Practice/Office Director and Principal




Insights

![Medical Devices, Implants & Surgical Tools [MCE]](/sites/default/files/styles/hero_purple/public/media/images/GettyImages-1182458826.jpg.webp?itok=Wo4S_-2U)


